PERIODIC CLASSIFICATION OF ELEMENTS
1. An orderly arrangement of elements such that those elements having similarity in physical and chemical properties occur at regular intervals is called periodic classification of elements.
2. Elements are first classified into metals and non-metals. It failed because there is no perfect metal and non-metal. Those having both metallic and non-metallic characters are called metalloids. But the term metalloid is not clearly defined.
3. Prout’s Hypothesis (1815): At.Wt. of every element is the Integral multiple of the At. Wt. of H. He thought H is the building unit of every atom.
4. Thomos Thomson supported Prout’s Hypothesis.
5. In 1817 J.W. Dobereiner proposed the LAW OF TRIADS. According to him, if chemically similar elements are arranged in threes in the increasing order ofA t.Wts., the average At.Wts. Of 1 and 3 elements is nearly equal to the At. Wt. of the second element. It failed because –
a) Not applicable to all chemically similar elements.
b) It is not a law, it is a mere relationship between the At.Weights of chemically similar elements.
NOTE: He is the first man to classify the elements basing on Atomic Weights.
6. In 1862 Newland proposed the Law of Octaves as follows:
If elements are arranged in the increasing order of their At.Weights, those having similarity in P and C properties will reoccur at intervals of 7″ ie., every element will resemble with its 8th element. This classification failed as
a) It is more applicable up to Ca.
b) It has not shown any place for the newly found out elements.
c) Position of ‘H’ is not explained.
NOTE: He is the first man to introduce the concept of periodicity.
7. In 1870, Lothar Meyer said “The physical properties of elements are periodic functions of their At.Wts. Atomic volume curves are drawn between atomic volumes and At. Wts.in which –
a) Alkali metals occupy peaks.
b) Transition metals occur on trough.
c) Halogens occupy ascending portions and alkaline earth metals occupy descending portions.
d) C, Al, also occur as troughs.
Likewise periodicity was observed in other physical properties like refractivity MP. Colour, etc. It failed
for Sp. gravity.
8. In 1869 D.I.Mendeleev, a Russian Scientist proposed his periodic law “The P & C properties of elements are periodic functions of their At. Weights”.
The meaning of the above periodic law is if elements are arranged in the increasing order of their At. Weights, those having similarity in P & C properties will reoccur at regular intervals.
a) At the time of Mendeleev 63 elements were known.
b) Lightest is H and heaviest is U
c) Vertical columns are groups and Horizontal rows are periods, 8 groups and 7 periods.
d) From 4th period every group is divided into 2 sub-groups except 8th group.
e) In 8th group 1, 2, 3 & 7 periods have no elements. Each one of 4, 5 & 6 periods have 3 elements. They are called transitional triads.
f) Beneath the table 14 elements are kept in a single row and they are named as Rare earths. Since they are rarely present in Earth crust.
NOTE: In the modern periodic table f-block elements are shown beneath the table separately, but they should not be referred as rare-earth because elements after Uranium are artificially made.
g) Third period elements are called typical elements and those resembling them are called normal elements.
h) A family of elements are called CONGENERS.
i) 2 & 3 period elements show diagonal relationship and 1st element in every group differs from rest.
Advantages of Mendeleev table:
1. Correction of Atomic Weights of elements. Be, In, U, Ir, Cs, Ce, Pt, Os, etc.
2. Leaving gaps promoted research. He left many gaps and predicted the existence of elements in 5 gaps and guessed the properties of 3 elements also .
3).Group numbers indicate the valency of the elements.
4) Anomalous pairs: Exchanged the positions I after Te, Ni after Co.
NOTE: Two more corrections were made afterwards – K after Ar, Pa after Th .
Demerits: The only and major defect in Mendeleev periodic table is classifying the elements on At.Weights. It lead to the following disadvantages:
1. Division of sub-groups, position of rare-earths, position of 8th group, isotopes, isobars, hydrogen. it is not properly explained.
2. Too much stress on valency.
3. Defective periodic law. So only anomalous pairs.
4. He failed to explain the reason for diagonal relation and also failed to explain why it is applicable up to Boron.
5. He failed to give a reason for the dissimilar behavior of the 1st elements in the group.
The following are predicted by Mendeleev and they are discovered as follows
Eka Boran – Sc (Wilson – 1879); Eka Aluminum – Ga, by Lecoq de boisbandran. – 1875; Eka silicon- Go-Winkler-1886;Eka Manganese – Tc by Perrier and Segre-1927; Dvi Manganese – Re (1925)
Short form of table or modified Mendeleev table:
1. In this inert elements and transuranic elements are also included.
2. It contains (8 +0) i.e., 9 groups. 6th and 7th periods include Lanthanides and actinides respectively, but they are shown separately. It contains 4 anomalous pairs. Co & Ni; Te & I; K & Ar; Th & Pa.
9. Long form of Periodic table or Bohr’s table or Mosley’s table or Modern periodic table:
a) Modern periodic law: The Physical and Chemical properties of elements are periodic functions of their electronic configuration in the valency shell.
b) Mosley’s Periodic law: “The Physical and Chemical proper ties of elements are periodic functions of their atomic numbers”. Since Mosley is the discoverer of Atomic number, it is called Mosley’s periodic table.
1. Elements are arranged in the increasing order of their At. No.
2. 7 horizontal rows – periods; 18 vertical columns groups:
1st period ————– Short, discontinuous – 2 elements.
2nd & 3rd periods —- Short, discontinuous – 8 elements.
4th & 5th periods —– Continuous – 18 elements.
6th period————— Longest continuous with 32 elements.
7th period————— Long continuous but incomplete-19 elements.
3.Lanthanides & actinides are present in 6th and 7th periods respectively, but shown separately beneath the table.
4. First 3 periods are made discontinuous to show a proper place for the elements having electrons in ‘d’ sub-orbit (start after Ca).
5. All the elements belonging to a group will have same electronic configuration (E.C) in valency shell.
c) Merits:
1. It is based on E. C. So inclusion of a set of elements in a group is properly justified.
2. There is no division into sub-groups.
3. Isotopes occupy same place since they have same At.No.
4. 2nd period elements differ from rest of elements in their group because they have 2 electrons in the penultimate shell while the others have more than 2 electrons.
d) Demerits:
1. ‘He’ is a’s’ block element, but kept in ‘p’ block.
2. Inclusion of ‘H’ along with Alkali metals is justified in terms of E.C., but not in terms of properties.
3. Lanthanides and actinides are shown separately.
NOTE:
‘f’ block elements will have same E.C. in ultimate and penultimate shells. So they show greater similarity. They can be separated only by physical methods like solvent extraction, centrifugation and chromatography.
10. Classification: There are two ways of classifying elements depending on the electronic configuration – 1. Division into s, p, d, f blocks.
2. Division into inert, normal transition and inner transition elements.
s block——————— IA & IIA. They ate all metals except ‘H’.
p block——————– 3A to 7A and O group. There are metals, nonmetals and metalloids.
d block——————– 1B to 7B and 8th group. They are all metals.
f block——————— Lanthanides & Actinides
3. Division into blocks: The last electron entering into the atom is called differentiating electron. Depending upon sub orbit occupied by the differentiating electron they are classified into s, p, d, f blocks. They have 2, 6, 10 & 14 groups respectively.
Second classification:
a) Inert elements:
1. They have s2 p6 configuration in valency shell except He (1s2 )
2. All electron containing sub-orbits are complete. All orbits are complete (Bohr-bury rule).
3. They are chemically least reactive.
4. These are called as bridge elements because they explain sudden change from Halogens to Alkali metals. They are also called Aerogens as they are found in air.
b) Normal elements:
1. Incompletely filled last orbit.
2. They have incompletely filled s or p sub-orbits in valency shell. General E. C. is ns1-2 np0-6. This group includes s & p block except p6 group. These are highly reactive because by losing, gaining or sharing belectrons they can get s2 p6 configuration.
c) Transition elements:
1. Incompletely filled last and penultimate orbits. They exhibit variable valency.
2. Incompletely filled ‘d’ sub-orbit in penultimate shell. General E.C. is (n-1)d1-10 ns1-2.except (n-1)d10 ns2. Zn, Cd, Hg are not transition elements by definition but they are by properties.
3. All’d’ block elements are transition elements by properties.
d) Inner transition elements:
1. Last, penultimate, anti-penultimate orbits are in complete.
2. They have incompletely filled ‘f’ sub-orbit in the anti-penultimate shell.
Their general E.C. is (n-2)f1-14 (n-1) d0-1ns 1-2except (n-2)f14(n-1)d10 ns². Lu and Lr are not inner transition elements by definition, but they are by properties. All ‘f’ block elements are inner transition elements by properties.
11. Periodicity in properties:
a) Atomic Radius: The term atomic radius is ambiguous. Actually single bond covalent radius is used for atomic radius.
b) Covalent Radius: It is the half of the inter nuclear distance between two covalently bonded homo atoms in solid state.
Sometimes atomic radius is calculated by measuring volume of 1 mole of substance at its M.P.
c) Factors influencing A.R:
i) As electron containing orbits increases, A.R increases.
ii) If E.N.C(effective nuclear charge) increases, A.R. decreases. E.N.C. means the actual No. of protons attracting the valency electrons.
a) In case of atoms E.N.C. is equal to number of valency electrons.
b) In a group from top to bottom A.R. ↑. In a period from left to right A.R.↓ up to halogens and suddenly increases to inert elements.
iii) Across the periods of transition elements, the change in A.R. is less when compared to normal elements because the differentiating electron enters into the penultimate shell. It is still less across the periods of inner transition element because the differentiating electron enters the anti-penultimate shell.
d) Ionic radius:
i) Always the size of cation is less than the size of corresponding neutral atom. When one or more electrons are removed from the atom No. of electrons are less, No. of protons are relatively more, E.N.C. increases, size decreases. Eg: Size of Na+ ˂ Na. As the magnitude of charge of cation increases, size gradually decreases. Size of Mg+2˂ Mg+ ˂ Mg.
ii) The size of anion is always more than the size of the corresponding neutral atom. Eg: Size of C1-1 > Cl. As the magnitude of charge of anion increases, size gradually increases. Size of O-2 ion more than O-1ion.
– 6 –
In case of isoelectronic ions as No. of protons (nuclear charge) increases, size decreases.
| C-4 | N-3 | O-2 | F-1 | Na+1 | Mg+2 | Al+3 |
| 10e | 10e | 10e | 10e | 10e | 10e | 10e |
| 6p | 7p | 8p | 9p | 11p | 12p | 13p |
From C-4 to Al+3 number of electrons are same but number of protons are increasing so size decreases in the same order.
Similarly size of Zn+2(28e&30p) ˂ Cu+1(28e&29p).
e) Vander Walls Radius: It is half of the internuclear distance between two adjacent, non-bonded homo atoms in solid state.
NOTE: The minimum approachable distance between two non bonded atoms is equal to sum of their Vander Waals radii.
f) Ionisation Potential: The amount of energy needed to remove an electron from a neutral gaseous atom is called I.P Or it is the amount of energy needed to convert a neutral gaseous atom to unipositive gaseous ion.
It is measured in K.Cal/mole or ev/mole. 1ev/mole = 23.06 K.Cals/mole.
Factors influencing I..P:
1. As A.R ↑ I.P ↓ because shielding or screening effect ↑.
2. I.P. of s >p >d> f electron because penetrating effect of s > p> d> f.
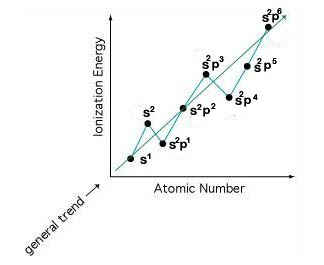
3. 1.P of elements with completely filled or half-filled Sub-orbit is more. IIA and VA elements have high I.P.
In a group from top to bottom I.P ↓, In a period from left to right I.P. ↑ with exceptions at Boron and Oxygen family.
4. Among all the elements highest I.P for ‘He’ and lowest I.P. for ‘Fr.’
5. In any period I.P. is as follows:
7. Amount of the energy needed to remove an electron from a uni positive gaseous ion is called 2nd IP. or it is the amount of energy needed to convert a unipositive gaseous ion into dipositive gaseous ion.
Always I1 < 12 < 13 < 14.
| Element | First I.P | Second I.P | Third I.P | Fourth I.P | Fifth I.P | Sixth I.P |
| Na | 5.1 | 47.3 | 72 | 98.9 | 138.5 | |
| Mg | 7.6 | 15 | 80 | 109 | 141.4 | 156.9 |
| Al | 6.0 | 18.8 | 28.5 | 120.1 | 153.5 | 190.4 |
| Si | 8.2 | 16.3 | 33.5 | 45.2 | 166.1 | 203.8 |
Using the I.P values of an element we can guess what type of cation it can form during chemical reaction.
The difference between I1 and I2 values is more for Na, SO it prefers unipositive ion.
Electro negativity: It is the tendency of an atom of an element in a molecule to attract shared electron pair towards itself. It is a relative property. Linus Pauling determined these relative values.
i) Among all the elements ‘F’ has very high EN value and ‘Fr’ has very low value
ii) Pauling formula:
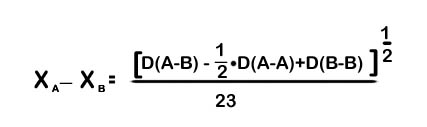
D(A-B), D(A-A) & D(B-B) are experimental bond energies.
iv) According to Mullikan
E.N = (Ionisation potential + Electron Affinity).1/2
v) X (Mullikan) = 2.78 x (Pauling)
Factors:
1. If A. R.↑ E.N. ↓.
2. As E.C. approaches near to s2 p6 E.N value increases. In a group from top to bottom E.N. decreases since A.R increases. In a period from left to right E.N. ↑ up to halogens and suddenly decreases to inert elements whose value is not assessable. The increase is, because (a) A.R. decreases (b) E.C. approaches to s2 p6.
3. In Boron family from Al to Ga E.N.↑. In Carbon family from Si to Ge. E.N. value remains same. It is because of less shielding effect of’d’ sub-orbit electrons in penultimate shell.
Electron affinity:
It is the amount of energy released when an electron is added to a neutral gaseous atom (or)
It is the amount of energy released when a neutral gaseous atom is changed to uninegative gaseous ion. E.A. of X (g) = I.P of X-1 (g). It is measured in electron volts.
– 8 –
If A.R. increases E.A decreases.
Electron affinity of IIA and VA is almost Zero due to completely filled and half filled sub-levels.
As E.C. approaches to s2p6 E.A. increases. Trends similar to E.N. except in 3 cases.
E.N. of Nitrogen > P but E.A of Nitrogen < P.
E.N. of Oxygen > S but E.A of Oxygen < S.
E.N. of Fluorine > Cl but E.A of Fluorine < Cl.
E.A values of N, O, F are misleading. It is actually those elements that readily change into anions compared to P, S, Cl. respectively.
2nd Electron affinity: It is the amount of energy needed to add an electron to a uninegative gaseous ion. (2nd E.A. is the amount of energy needed to convert a uninegative gaseous ion into dinegative gaseous ion).
x– (g) +e– + E2 → x-2(g)
Except 1st E.A. the other values are endothermic.
1. Reason for diagonal relationship:
From left to right E.N. value increases and from top to bottom E.N. value decreases so Li-Mg, Be-Al, B-Si are having nearly equal E.N. values that is why they resemble with each other.
2. Valency:
a) It is the combining capacity of the atom of an element in a molecule.
b) There are two standards for valency – Hydrogen standard which increased from 1 to 4.
NaH, CaH2 AlH3, SiH4.
Oxygen standard which increased from 1 to 8
Na2O, Cao, A1203, Si02, P205, SO3, Cl207, 0s04. Valiancy of ‘Os’ is 8.
c) Latest definition of valency is “No. of unpaired electrons in the ground or excited state of an atom of an element”.
1s2, 2s2, 2p6, 3s2, 3px2, 3py1,3pz1 —————Ground state —-valency = 2.
1s2, 2s2, 2p6, 3s2, 3px1, 3py1,3pz1 , 3d1 —————Excited state —-valency = 4.
1s2, 2s2, 2p6, 3s1, 3px1, 3py1,3pz1 , 3d1,3d1 —————Excited state —-valency = 6.
d) In a group all the elements will have same valency Ex: Li, Na, K, Rb, Cs, Fr.
e) In a period valency gradually increases from 1 to 4 and then decreases to 1 with respect to H standard..
f) In a period valency gradually increases from 1 to 7 w.r.t. Oxygen standard.
g) An element will form only covalent bonds in excited state.
– 9 –
h) Generally higher valency is more stable than lower valency.However there are 4 exceptions T1, Sn, Pb and Bi.
For ‘T1’ valency 1 is more stable than 3.
For ‘Sn’ valency 2 is more stable than 4.
For ‘Pb’ valency 3is more stable than 4.
For ‘Bi’ valency 3 is more stable than 5.
The reason is’s’ electron pair in these elements will not get unpaired easily. It is called Inert pair effect.
3. Metallic Character:
a) In a period from left to right ionisation energy gradually increases hence Metallic Character gradually decreases and non-metallic nature gradually increases.
b) In a group from top to bottom ionisation energy gradually decreases hence metallic character gradually increases.
Reducing and oxidising nature:
a) In a period from left to right reducing property gradually decreases and oxidising character gradually increases. It is because ionisation energy gradually increases.
Ex: Na, Mg and Al are strong reducing agents. Si is a mild reducing agent P is both strong oxidising and strong reducing S is weak oxidising and reducing agent. Cl is a strong oxidising agent.
b) In a group from top to bottom the capacity to act as reducing agent increases and as oxidising agent decreases. It is because ionisation energy decreases.
5. Basic and Acidic nature of oxides:
In a period from left to right Basic nature decreases and acidic nature increases.
Na2O, Mgo are Basic. A1203 is Amphoteric. Sio2 ,P203 ,S02 ,C1207 are Acidic.
In a group:
a) If oxides are basic their basic nature increases down the group. b) If oxides are acidic their acidic nature decreases.
6. Lanthanide Contraction: Shielding effect of f sub-orbit is less.
From Ce to Lu the differentiating electron is entering into ‘f’ sub-orbit. Nuclear attraction gradually increases. The size of the atom in the third transition series is almost same as the atom in the second transition series. The radius of Zr(1.45) for example, is almost equal to the radius of Hf(1.44), and the radius of Nb is almost equal to the radius of Ta, and so on. It is called Lanthanide contraction and it is prominent up to VIB group.
– 10 –
7. d-d transition: Shifting of electron/s between the lower energy ‘d’ orbital to a higher energy ‘d’ orbital by absorption of energy and vice versa. Due to d-d transition some compounds of transition elements are coloured.
8. Transition elements and their compounds act as catalysts. eg. Nickel acts as catalyst in hydrogenation of oils.
9. The catalyst is the substance which can alter the rate of reaction, whereas the promoter is the substance which is added during the reaction will increase the efficiency of the catalyst. In the manufacture of ammonia by Haber’s process molybdenum is a promoter to the catalyst is iron.
10. Most of the transition elements show paramagnetic behaviour. The unpaired electrons in (n-1) d orbitals are responsible for the magnetic properties. The paramagnetic character of the transition metals increases on moving from left to right as the number of unpaired electrons increases from one to five.
11. Transition metals have very similar atomic sizes. One metal can easily replace the other metal from its lattice to form solid solution (alloy). Transition metals are miscible with one another in the molten state. The molten state solution of two or more transition metals on cooling forms alloy.
12. The common stable oxidation state of lanthanides is +3.
13. The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are synthetic, unstable and decay radioactively into other elements.
14. Atomic radius of the atom of an element depends upon-the type of bonding it is involved in the compound – its oxidation state in the compound – its coordination number in the compound and the number of bonds it is forming in the compound.
15. Metallic or crystal radius is half the inter nuclear distance the adjacent atoms in a metal crystal.
16. The Vander Waals radius is equal to one half the distance between two unbonded atoms when the electrostatic forces between them are balanced. In other words, it is half of the closest distance between two atoms that aren’t bonded or within the same molecule. It is nearly 40% more than covalent radius.
17. As the number of bonds between similar atoms increases covalent radius decreases.
| Type of bond | Bond length | Covalent radius |
| C-C | 1.74A0 | 0.77A0 |
| C=C | 1.34a0 | 0.67A0 |
| C≡C | 1.20A0 | 0.60A0 |
18. In any period halogens will have maximum Electron affinity as they need only one electron to get inert gas configuration. Chlorine has the highest electron affinity among the elements.